|
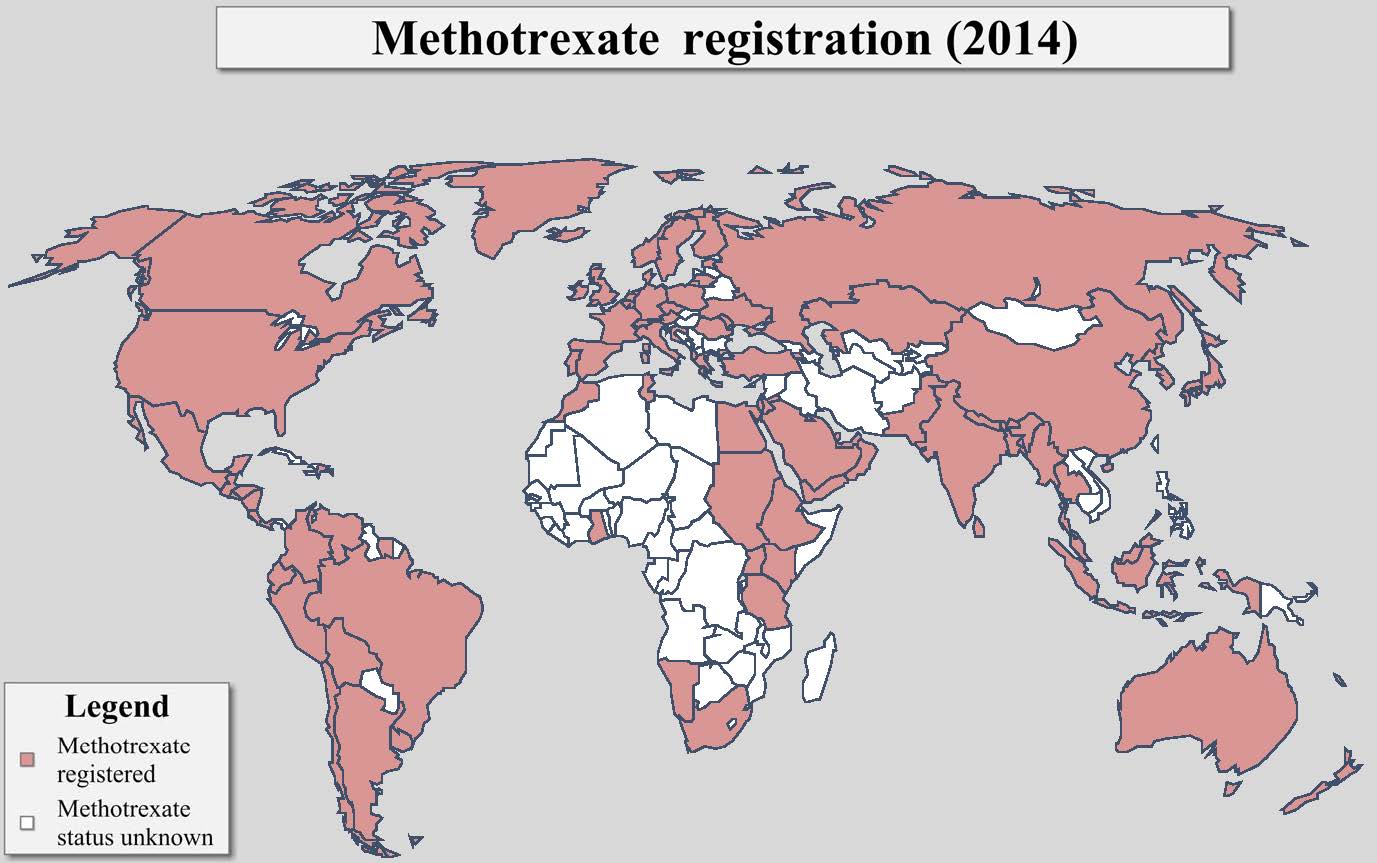
This map was originally created by the
Population Council in 2002. In 2009 and 2014, Ibis Reproductive Health conducted a
follow-up assessment of global methotrexate registration and incorporated those
findings accordingly. |
| |
Overview and history
Since 1953, methotrexate has been available in the United States as a treatment for cancer. A chemotherapeutic agent, methotrexate has also been used since the 1980s to treat ectopic (extra-uterine) pregnancies. However, when the political environment in the US delayed the approval and availability of mifepristone as a medication abortion regimen, providers and researchers began to investigate the possibility of expanding the use of methotrexate to early pregnancy termination. In 1993, investigators initiated the first study using low-dose methotrexate in combination with misoprostol for early abortion. Subsequent studies have shown that the methotrexate/misoprostol regimen constitutes an effective method of terminating early pregnancies.
Methotrexate
availability worldwide
As of 2014, methotrexate had been registered in more than 70 countries worldwide. In most countries, methotrexate is registered as a chemotherapeutic agent and/or for the treatment of ectopic pregnancies. In many settings, the use of methotrexate for medication abortion is “off-label” meaning that the methotrexate is being used for an unapproved purpose or indication. However, there is a body of research that demonstrates the safety and efficacy of the methotrexate/misoprostol medication abortion regimen and evidence based protocols have been established. Thus it is permissible for clinicians to use methotrexate for this purpose and professional organization guidelines also allow this practice.
Mechanism of action of methotrexate
Methotrexate is an anti-metabolite. By blocking the enzyme dihydrofolate reductase, methotrexate inhibits the production of thymidine, a requirement for DNA synthesis. Methotrexate interferes with cell growth and specifically interferes with rapidly dividing cells. Conditions that produce rapid cell division include neoplastic disease, autoimmune diseases, and pregnancy. Methotrexate primarily affects the cytotrophoblast and inhibits, rather than weakens, the implantation process.
Methotrexate is used in conjunction with misoprostol (common brand name is Cytotec®). Misoprostol is an analog of prostaglandin E1. By interacting with prostaglandin receptors, misoprostol causes the cervix to soften and the uterus to contract, resulting in the expulsion of the uterine contents.
Methotrexate and misoprostol protocol
The most common evidence-based protocol begins with either the intramuscular injection (50 mg/m²) or oral administration (50 mg) of methotrexate (Day 1). Three to seven days later the woman self-administers 800 micrograms of misoprostol vaginally at home. Follow-up with a provider occurs approximately one week after the methotrexate administration (Day 7). If the abortion has not occurred (as determined by vaginal ultrasound examination) the dose of misoprostol is repeated and the woman returns for final evaluation four weeks after the methotrexate administration (Day 28). However, if at the first follow-up visit (Day 7), embryonic cardiac activity is noted on ultrasound, the woman is given an additional dose of misoprostol and asked to return on Day 14. If the abortion is not complete on either the Day 28 or the Day 14 visit, vacuum aspiration is typically performed.
Efficacy
Approximately 95% of women will have a complete abortion when using methotrexate/misoprostol up to 49 days' gestation. Medication abortion completion rates with the methotrexate and misoprostol regimen decline with increasing gestational age, with completion rates of approximately 82% between 50 and 56 days' gestation.
Although the overall efficacy of the methotrexate/misoprostol regimen is similar to that of mifepristone and misoprostol within 49 days' gestation, timing of completion is quite different. For approximately one fifth of patients, the abortion will occur up to four weeks after misoprostol administration. For women who do not experience a complete abortion an aspiration intervention may be required.
Ongoing pregnancy occurs in fewer than 1% of cases. However, methotrexate is a known teratogen and in utero exposure to the medication leads to severe fetal abnormalities and/or fetal death. Thus if a woman experiences an ongoing pregnancy after taking methotrexate an aspiration termination is highly recommended.
Eligibility
Most women with an early pregnancy can use the methotrexate and misoprostol regimen. Although the regimen is most effective in the first seven weeks of pregnancy, women with pregnancies of up to 9 weeks; gestation may still use the methotrexate and misoprostol regimen safely and effectively. Accurate dating of the pregnancy is critical and can occur through either clinical assessment or ultrasound. Methotrexate has demonstrated efficacy in treating ectopic pregnancies and is thus the preferred regimen for women with suspected extra-uterine pregnancies. If the use of methotrexate and misoprostol results in an incomplete abortion, aspiration intervention may be necessary. Women considering the methotrexate and misoprostol regimen should we willing to undergo a vacuum aspiration, if indicated.
Contraindications
There are a number of contraindications to methotrexate/misoprostol use. These include: a history of allergy or intolerance to either methotrexate or misoprostol; coagulopathy or current severe anemia; acute or chronic renal or hepatic disease; acute inflammatory bowel disease; or uncontrolled seizure disorders. Further, if an intrauterine device (IUD) is present, the device must be removed before a methotrexate/misoprostol termination is initiated.
To date, no data is available on the effect of folate supplementation on the efficacy of the methotrexate/misoprostol regimen. Generally, patients are advised to discontinue the use of folate supplements for one week after methotrexate administration. Women may also be advised to discontinue consumption of leafy green vegetables, beans, and organ meats for two weeks after methotrexate administration. However, no studies have evaluated the necessity of dietary modifications.
Effects, side effects and complications
Effects
Abdominal cramping and vaginal bleeding are hallmarks of the abortion process itself. Many women and clinicians report cramps and abdominal pain similar to those associated with a heavy menstrual period. Vaginal bleeding can vary significantly in both duration and severity, and many report that the bleeding resembles a heavy period or a spontaneous miscarriage. Bleeding can last for weeks; the mean duration of bleeding is 14 to 21 days.
Side effects
Side effects of methotrexate include nausea, vomiting, diarrhea, fever or chills, headache, dizziness, and oral ulcers. Side effects of the misoprostol include nausea, vomiting, diarrhea, fever, and chills. In most cases, side effects can be managed with appropriate counseling and symptomatic treatments, such as oral analgesics (ibu profren) for pain.
In the high doses used in the chemotherapy regimen, methotrexate exposure during pregnancy has been associated with numerous fetal malformations. Several case reports indicate that methotrexate may have teratogenic effects in cases of incomplete abortion. Women electing to use the methotrexate/misoprostol regimen should be informed of the teratogenic effects of methotrexate and should be counseled on the importance of aspiration completion in the event that the medication abortion is unsuccessful.
Complications
When administered in the first seven weeks of pregnancy, the overall failure rate is approximately 5%. Most of these patients require an aspiration abortion to resolve an incomplete abortion, end a continuing pregnancy, or control bleeding. Extremely heavy or prolonged bleeding is rare; less than 1% of women required intervention for heavy bleeding. Ongoing pregnancy occurs in less than 1% of cases.
Acceptability
Studies on the acceptability of the methotrexate/misoprostol regimen have found that the majority of women found the method satisfactory. Further, the majority of women reported that they would both choose the methotrexate/misoprostol method again and recommend the method to others.
Additional uses of methotrexate
Methotrexate is commonly used to treat neoplastic diseases, rheumatoid arthritis, and psoriasis. Methotrexate is also used to treat ectopic pregnancies, Crohn's disease, systemic lupus, and severe asthma.
For a list of sources consulted for this section of the website, please visit our reference section.